 As readers may have guessed from previous posts, my brewing interests are minimally conventional. Fortunately, the Basic Brewing Radio podcast seems to regularly expand well beyond the usual “fermented malt flavored with a tisane of hops” thing (I need to try to make my own “Ginger Beer Plant” from scratch one of these days…). A couple of weeks ago, they did an episode covering an experiment on aeration methods which was very interesting. It does my ego good to know that I correctly guessed how the results would turn out. You can get a copy of the nice write-up of the experiment itself here, but here’s the simple version:
As readers may have guessed from previous posts, my brewing interests are minimally conventional. Fortunately, the Basic Brewing Radio podcast seems to regularly expand well beyond the usual “fermented malt flavored with a tisane of hops” thing (I need to try to make my own “Ginger Beer Plant” from scratch one of these days…). A couple of weeks ago, they did an episode covering an experiment on aeration methods which was very interesting. It does my ego good to know that I correctly guessed how the results would turn out. You can get a copy of the nice write-up of the experiment itself here, but here’s the simple version:
Category: Brew
(Tap Tap Tap) Is this thing on?…
Move is underway. Here’s a quick update / test.

On the way home (to Texas, that is, with a load of furniture from House v1.0 in Idaho) we adopted a co-dog. Now Cornelia isn’t the only official dog in the house.
Yeah, I know, pretty frivolous stuff. What do you want – this is mainly a test post. You guys ARE seeing this post, right? (Please comment and let me know). I’ve finally found and jumped through the hoops necessary to migrate the bigroom.org website over to the new host. It’s also running on the old server in House v1.0 on the DSL line as well, but this post isn’t getting put on it. If I’ve done everything correctly, hosting for bigroom.org should now be handled by Eskimo North. Just a few other minor tweaks and I’ll be set to return to House v1.0 and shut down the old server so I can move it down here to Texas.
I’ve gone from about 4400ft elevation down to about 250ft, but the plastic bottles of Mountain Dew® Wine seem to have positive pressure, so it would appear there is still some live yeast left in there and the priming sugar is slowly doing its job. I’m pretty sure the benzoic acid is what’s slowing down the activity so drastically, but it hasn’t killed it off yet.
I’ve also got a couple of entries in mind already for the next The Giants’ Shoulders blog carnival, and hopefully some more crazed brewing project news to offer sometime. And, of course, the promised post on why benzoic acid works. Stay tuned.
Mountain Dew® Wine: Disappointment strikes!
<whine excuse=”obligatory”>Have I ever mentioned what a huge hassle it is to relocate from one abode to another 1600 miles away?…</whine>
 I’m finally back at House v1.0 where I can check on the progress of my Mountain Dew® Wine. It appears to have managed to ferment, in spite of the severe dose of preservatives in the stuff designed to prevent that from happening. It went somewhat slowly, but it’s gone from an original gravity of around 1.054 down to about 1.011 or so, suggesting about, say, 5-6% alcohol in the final product, which has faded to a pale, cloudy yellow color. Hopefully the cloudiness is from still-living yeast, which has now demonstrated that it is reasonably benzoic-acid-and-caffeine tolerant.
I’m finally back at House v1.0 where I can check on the progress of my Mountain Dew® Wine. It appears to have managed to ferment, in spite of the severe dose of preservatives in the stuff designed to prevent that from happening. It went somewhat slowly, but it’s gone from an original gravity of around 1.054 down to about 1.011 or so, suggesting about, say, 5-6% alcohol in the final product, which has faded to a pale, cloudy yellow color. Hopefully the cloudiness is from still-living yeast, which has now demonstrated that it is reasonably benzoic-acid-and-caffeine tolerant.
I fear I must report that the result is a crushing disappointment to me. It’s not very good. Worse yet, it’s not very bad, either. I was hoping that if it wasn’t surprisingly tasty that it would at least be shockingly awful in some interesting way so I’d have something entertaining to say about it here.
Actually, the adjective that comes to mind is “inoffensive”. If you would like to whip up a quick simulation of what I’ve got here, you might be able to do it like this: Take some citrus-flavored sparkling mineral water. Dilute it about half with (uncarbonated) distilled water. Then mix about 7 volumes of that with one volume of vodka. What I’ve got here is slightly sparkling, with a barely noticeable citrus flavor and little or no remaining sweetness. It’s a little surprising to me just how much of the flavor of Mountain Dew® apparently comes from its sweetness. Perhaps next time I try this (if there ever IS a “next time”…) I’ll have to mix regular and “diet” Mountain Dew® – with some bonus sugar to make up the difference, of course.
I’m not completely done here. I’m still going to dispense it into cleaned bottles with a little bit of sugar to prime it for full carbonation. Sanitized plastic soda-bottles of course – none of that snobby glass stuff for this here experimental drinkin’ substance! It may be high-class for “pruno”, but it’s sure as heck not Champagne™. (Besides, I want to evaluate reusing plastic bottles anyway – it’d be a lot easier to tell when there’s too much pressure and to let some of the pressure off if there is.) Plus, I need to take some of the still-live yeast and keep it alive. No point in developing a benzoic-acid-tolerant yeast strain and not keeping it!
On a related note, an article was mentioned on fark.com, saying that back in 1955, a scientist “proved” that it is not normally possible to get drunk on beer. Of course, he seems to have been referring to dilute mass-market bladderwash and his reasoning was that a typical human stomach cannot contain enough 3.7%-alcohol beer for a typical human to achieve a dangerous blood-alcohol level.
As usual, the articles (see here and here) go for the “a scientist says this” part but never bother to say WHERE the scientist says it – usually a real scientific publication.
A quick search of pubmed turns up a likely candidate:
Greenberg LA: “The definition of an intoxicating beverage.” Q J Stud Alcohol. 1955 Jun;16(2):316-25 (link goes to the pubmed entry, which has little more information that this).
I do believe it is a moral imperative that I get a copy of this article somewhere so that I may reference it later. Is there anyone out there reading this who might be able to get a copy of this paper somewhere for me? Please?…
SHENANIGANS! Caffeine is our FRIEND!
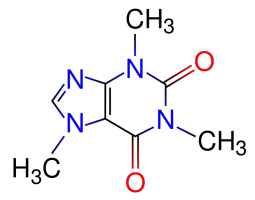 Our new Asylum has real internet finally now and we’re getting settled in. The Houston area here is one of the most hot and humid areas of the US. All hot and sweaty. So of course I’ve been advised that my favorite psychotropic substance – 1,3,7-trimethylxanthine [“caffeine” for party-poopers who aren’t into the fancier names] – is no longer my friend, because it’s a diuretic that’ll dehydrate me, right?
Our new Asylum has real internet finally now and we’re getting settled in. The Houston area here is one of the most hot and humid areas of the US. All hot and sweaty. So of course I’ve been advised that my favorite psychotropic substance – 1,3,7-trimethylxanthine [“caffeine” for party-poopers who aren’t into the fancier names] – is no longer my friend, because it’s a diuretic that’ll dehydrate me, right?
NO! Shenanigans! Caffeine is our FRIEND! And that stuff about it being a diuretic? CRAP! LIES AND SLANDER!
But don’t just take my word for it. After all, humans are a bunch of freakish multicellular soft-celled eukaryotes, and I normally focus on normal organisms like bacteria, archaea, and yeasts. So, let’s ask some real human-physiology type scientists and check out their official peer-reviewed findings:
Armstrong LE, Pumerantz AC, Roti MW, Judelson DA, Watson G, Dias JC, Sokmen B, Casa DJ, Maresh CM, Lieberman H, Kellogg M: “Fluid, electrolyte, and renal indices of hydration during 11 days of controlled caffeine consumption.”; Int J Sport Nutr Exerc Metab. 2005 Jun;15(3):252-65.
“[…]The following variables were unaffected (P > 0.05) by different caffeine doses on days 1, 3, 6, 9, and 11 and were within normal clinical ranges: body mass, urine osmolality, urine specific gravity, urine color, 24-h urine volume, 24-h Na+ and K+ excretion, 24-h creatinine, blood urea nitrogen, serum Na+ and K+, serum osmolality, hematocrit, and total plasma protein. Therefore, C0, C3, and C6 exhibited no evidence of hypohydration.[…]”
Armstrong LE, Casa DJ, Maresh CM, Ganio MS: “Caffeine, fluid-electrolyte balance, temperature regulation, and exercise-heat tolerance.” Exerc Sport Sci Rev. 2007 Jul;35(3):135-40.
“[…]This review, contrary to popular beliefs, proposes that caffeine consumption does not result in the following: (a) water-electrolyte imbalances or hyperthermia and (b) reduced exercise-heat tolerance.”
(Review article, apparently – Abstract on Pubmed)
Del Coso J, Estevez E, Mora-Rodriguez R: “Caffeine effects on short-term performance during prolonged exercise in the heat.” Med Sci Sports Exerc. 2008 Apr;40(4):744-51.
“[…]RESULTS: Without fluid replacement (NF and NF + CAFF), subjects were dehydrated by 3.8 +/- 0.3%[…]CONCLUSION: During prolonged exercise in the heat, caffeine ingestion (6 mg.kg body weight) maintains MVC and increases PMAX despite dehydration and hyperthermia. When combined with water and carbohydrate, caffeine ingestion increases maximal leg force by increasing VA (i.e., reducing central fatigue).”
(“NF” = “No Fluid replacement” – the “dehydration” mentioned here is due to exercising in the heat, and doesn’t appear to be related to whether the test subjects consumed caffeine or not)
Scott D, Rycroft JA, Aspen J, Chapman C, Brown B:”The effect of drinking tea at high altitude on hydration status and mood.” Eur J Appl Physiol. 2004 Apr;91(4):493-8. Epub 2004 Feb 11.
“[…]Several markers of hydration status were also taken immediately pre and post each condition, including measures of urine specific gravity, urine electrolyte balance (K+, Na+), and urine colour. None of these measures indicated a difference in hydration status as a result of the dietary intervention in either the control or tea condition.[…]”
(In this study, the tea was the only caffeine-containing substance involved. The study group’s caffeine came solely from the tea. The control group got no caffeine at all.)
Paluska SA: “Caffeine and exercise.” Curr Sports Med Rep. 2003 Aug;2(4):213-9.
“[…]It[caffeine] is relatively safe and has no known negative performance effects, nor does it cause significant dehydration or electrolyte imbalance during exercise.[…]”
Grandjean AC, Reimers KJ, Bannick KE, Haven MC.: “The effect of caffeinated, non-caffeinated, caloric and non-caloric beverages on hydration.” J Am Coll Nutr. 2000 Oct;19(5):591-600.
“[…]This preliminary study found no significant differences in the effect of various combinations of beverages on hydration status of healthy adult males.[…]”
Pubmed entry – full text available
See? Oh, I know what you’re going to say next – “But, like, dude! When I drink my Venti Mocha Crappucino [note: Link goes to “Foamy the Squirrel”, who is a bit of a pottymouth, ranting about the “Tall/Grande/Venti” nonsense. It amused me.] or a can of Jolt Ultra I have to take a major whiz a little while later! Isn’t that ‘cuz of the caffeine?” Well, no, it isn’t. It’s because you just drank a bunch of liquid. Duh.
So, you see, caffeine really is our friend. Be nice to caffeine. But don’t feed it to your yeast in the presence of benzoic acid because it’ll kill them. See? I managed to turn this into a segue back to the stuff I was talking about before the whole “buy a house in Texas” thing started interfering. Stay tuned…
Very brief post…
Sorry about having another brief bout of blogstipation. We’ve finally managed to close on a house and now we’re probationary Texans (y’all). I’ve been spending the last week+ driving back to SE Idaho (by way of Best Friends, since we now have room to hire a second dog to hopefully keep Cornelia the Laser Dog company. I’ve got to lug 3 cats (and one goldfish in a small fish tank) a distance of 1600 miles or so starting in about 6-8 hours. Wish me (good) luck.
After the weekend, I should have some time to get back to the real posts. The Mountain Dew Wine was virtually unfermented when I returned, even after sitting there 10 days, but since I’ve gotten back it’s started going. Still slowly – the bubbler spits out 3-5 bubbles ever 40 seconds or so – but it’s going. It’ll be interesting to see what I end up with. I wanted to do 2-3 posts on the effects of benzoic acid on yeasts (that’s the preservative in Mountain Dew®), and I would swear I had one or two others in mind. Oh, yes, and an update on getting a Magellan GPS replacement that can actually be used – they seem to have located a slightly lower-end model eXplorist that they can send me. I sent them back their “walled garden”-based Triton 500, so the replacement unit ought to show up next week, I think. Their service has been pretty good, at least.
“Antibiotic Susceptibility Testing by a Standardized Single-Disk Method”
Okay, one last post in the Classic Science Papers challenge before my time’s up:
Bauer AW, Kirby WM, Sherris JC, Turck M :”Antibiotic susceptibility testing by a standardized single disk method.” Am J Clin Pathol. 1966 Apr;45(4):493-6.
![Plates showing that Hops extract [like in beer] does inhibit certain kinds of bacteria Petri dishes containing bacteria, showing inhibition of growth by certain substances](http://www.bigroom.org/images/hops_plates.jpg) The “Kirby-Bauer” antibiotic susceptibility test is another standard method that you should cover in microbiology class. The method involves getting a pure culture of the bacteria you want to treat, and then growing it in a petri dish. By putting paper disks soaked with various anti-bacterial substances, you can identify which ones are most effective at killing (or at least stopping) the bacteria in question – for example if you’re trying to figure out what kind of antibiotic to give to the guy coughing up some unknown plague in your doctor’s office… The anti-bacterial substance that the paper is soaked in slowly diffuses into the area around it on the petri dish, getting more dilute the further it gets from the paper. You can then estimate how powerfully anti-bacterial the stuff is by how far from the paper the bacteria stop growing.
The “Kirby-Bauer” antibiotic susceptibility test is another standard method that you should cover in microbiology class. The method involves getting a pure culture of the bacteria you want to treat, and then growing it in a petri dish. By putting paper disks soaked with various anti-bacterial substances, you can identify which ones are most effective at killing (or at least stopping) the bacteria in question – for example if you’re trying to figure out what kind of antibiotic to give to the guy coughing up some unknown plague in your doctor’s office… The anti-bacterial substance that the paper is soaked in slowly diffuses into the area around it on the petri dish, getting more dilute the further it gets from the paper. You can then estimate how powerfully anti-bacterial the stuff is by how far from the paper the bacteria stop growing.
The authors here didn’t invent this trick. Not all antibiotic-susceptibility tests are “Kirby-Bauer” tests (the blurry picture there is of an experiment I did involving the beer ingredient hops, and is not a Kirby-Bauer test. Click the picture to go to my “Beer Cures Anthrax” post from long ago…). What this paper describes is a method that finally standardized this test. Instead of having to use multiple paper disks with different amounts of the same substance, the “Kirby-Bauer” test prescribes specific concentrations of each antibiotic, and specific nutrient agar formulations, and so forth, so that determining which antibiotic your mystery bug is best treated with can be done in a way that gives consistent results regardless of who is performing the test.
The method is regularly updated to account for new antibiotics, but is still referred to as the “Kirby-Bauer” antibiotic susceptibility test to this day. Incidentally, the American Society for Microbiology kindly hosts a reprint of this paper as a .pdf file, so you can read it yourself if you’d like.
(UPDATE 20110328: new URL for the reprint of the paper. Thanks, Alex S!)
“A small modification of Koch’s plating method.”
Only two more days for the Classic Papers Challenge, so if I’m going to get any more up, I’d better get my butt in gear.
Here’s a nice easy one:
The American Society for Microbiology has a translation available online. It’s only about a page-and-a-half of relatively large type – check it out.
There’s a trick we microbiologists use for growing bacteria. You make a solid (but wet) surface that contains whatever nutrients the microbe (bacteria, archaea, yeasts, mold spores…) you’re interested in need, and then you spread a diluted mixture of the microbe on it. The idea is that since the surface is solid the microbes can’t move around too much, and at any spot where a single cell starts initially, a whole pile of that cell and it’s genetically-identical (non-sexually-produced) clone-children will form until it gets big enough to see without a microscope. This cell-pile is called a “colony”, and you can poke or rub it with a sterile object, then stick the object into a sterile nutrient source. The end result is you have a “pure” culture of microbes that are effectively genetically identical. The solid material could be a lot of things – I’ve seen references to using slices of potato – though these days agar-agar gel mixed with nutrients is the preferred substance.
Koch (that is, Robert Koch of “Koch’s Postulates” fame, not Ed Koch the former mayor of New York City) used gelatin (so, hey, here’s another thing you can do with your expired Jell-O®). He apparently used to have a stack of shallow bowls, and had to use a special pouring device to carefully dump the gelatin into each stacked bowl in turn, then cover the works with a bell jar in order to keep stuff from falling into them from the air and contaminating them.
This was kind of a pain to work with, so some clever guy named Julius came up with a modification of this method in 1887, using pairs of shallow dishes, one slightly larger than the other so that it could be turned upside down to use as a lid. Then, you don’t necessarily need the bell jar, and you don’t need to stack them so they’re easier to pour.
 Julius Robert Petri’s idea was so useful that we still use it today. Oh, yeah, and they named the dish-and-lid combination after him.
Julius Robert Petri’s idea was so useful that we still use it today. Oh, yeah, and they named the dish-and-lid combination after him.
How’s that for a “classic” paper?
Meanwhile, my “Mountain Dew® Wine” project is turning out to be substantially more educational and fascinating than I’d hoped. There seems to be a decent amount of information available on how benzoic acid affects yeasts. I intend to turn that into a post later, but first I’ll try to find at least one more old paper to post before tomorrow is over…
Another cheatin’ “Open Thread” and random stuff
No single topics to dominate a post today. I’m in a hurry (as usual lately) and have very little time. Tomorrow morning I’m back on the 1600-mile route back to Southeast Texas, hopefully to sign the closing paperwork on the house we’re trying to buy.
My Mountain Dew® Wine appears to be still sitting there after several hours. Either the benzoic acid is still inhibiting the fermentation (in which case it’ll go REALLY slowly) or the yeast is just in shock or something. We’ll see how it looks in the morning. I’ll leave it for a week or so anyway to see how it does. Meanwhile I’ll refrigerate the other batch of yeast culture until I get back. If I have to develop my own strain of “Mountain Dew Yeast” I will, dagnabbit!
I did get a chance to go for a quick walk in the Big Room on the way back from some errands yesterday, so it gives me an excuse to play with the wordpress map plugin again (RSS feed readers: the map doesn’t get inserted there. Please check out the interactive map at the blog’s website here.) Comments on the map (or anything else, really – I DID say “Open Thread” after all) are encouraged – what do you think? I’d like to do some audio content for points on a map at some point, too. Maybe some video.
Lava Rock Walk [height=560;width=560]
If anyone’s bored enough to want to see how I get from Southeast Idaho to Southeast Texas, I can post a map of that tomorrow, too…
I’m not a real hillbilly, but I play one on the internet…
My first “Will It Ferment” project is now in process.
See, I’m stuck with a somewhat unpredictable schedule and a need to travel frequently, cramped spaces to work in at the moment, and a streak of gustatory perversion that I just can’t help an urge to rebel against purists at the moment.
Quick bit of background on that latter statement: I’ve noticed that people seem to think there are only two-and-a-half kinds of “real” non-commercial fermented beverages. There’s beer (which is apparently defined as a strong tisane of hops, flavored by mixing it into fermented malt), there’s wine (which is always made of grapes of course), and out on the fringes of respectability is Mead (“Honey wine”) which seems to be slowly gaining some acceptance as a mildly exotic brew. The attitude is that anything else you might want to brew (say, a beer flavored and preserved with something besides hops, or a wine made out of anything but grapes) is probably some quaint “country” (i.e. hillbilly) thing for people who either live too far from civilization or are too poor to just buy a “real” beverage, or are too ignorant to know the difference. Either that, or it’s just some desperate attempt to make something to get drunk on. Might as well be making pruno.
The attitude kind of annoys me, so I’m trying to make a sort of “free person’s pruno”. I figure if the end result is as palatable as it could be, it’ll probably resemble “Zima®”. Uh, no, I don’t expect it to be great – this is merely an experiment. My must has an Original Specific Gravity (“O.G.”) of approximately 1.054, about the same as most lagers start out. As I write this, my gallon of must is on the stove in a stainless steel pot. I’m going to try to heat it to boiling and let it boil for a few minutes in hopes of driving off much of the benzoic acid in it, so as not to inhibit the yeast fermentation.
 To your right, you should see the ingredients used in this project. Yes, those two bottles in the background are there on purpose. I’m trying to make…Mountain Dew® Wine.
To your right, you should see the ingredients used in this project. Yes, those two bottles in the background are there on purpose. I’m trying to make…Mountain Dew® Wine.
Here’s the plan:
- Last night, I took a couple of clean glass 1 Qt milk bottles, rinsed them with iodophor solution to sanitize, let them dry. Then, I put about 12 ounces of tap water (unchlorinated) into one and dumped the contents of both yeast packets you see in the picture into it to rehydrate.
- The yeast packets were opened over a year ago – I don’t even recall what I did with the tiny amount of yeast I poured out. They’ve been sitting in the ‘fridge since then. What’s more, they had expired in December of 2006 to begin with.
- The two yeasts (Red Star Montrachet and Red Star Flor Sherry) were both mixed into the same container in hopes that I could encourage a yeast orgy, giving me as much genetic diversity as possible from the two strains and maximizing the chance that I’d be able to get a culture which can grow in flat Mountain Dew and whatever benzoic acid (from Sodium Benzoate) might remain in it. I had read that V8 juice was actually an excellent medium for inducing yeast sporulation. Since sporulation occurs as a result of yeast cells mating, I made a huge leap of logic towards thinking the V8 might maximize my chances of getting some yeast mating going on.
- After a few minutes to rehydrate, I dumped the 12-ounce can of V8 into the bottle and shook well. I also crushed up a children’s chewable vitamin (see bottle at lower-left) and a small portion (perhaps 1/5) of a capsule of the L-Arginine as a nitrogen source and added them as well. I then poured it back and forth between the two bottles a few times to aerate, then split the mixture between the two bottles, capped loosely, and went to bed.
- As of this morning, fermentation was obviously occurring in the mixture, so sitting in my fridge open for over a year hadn’t killed off ALL of the cells. I opened the bottles and swirled to re-aerate, and added about a tablespoon of “corn sugar” (glucose a.k.a. dextrose) to wake the yeast cells back up. Since then, I’ve been pouring a bit of Mountain Dew right out of a third bottle into each of the yeast starter bottles every couple of hours. The amount of bubbling I see suggests to me that fermentation is still going on (and is not just from the carbonation of the Mountain Dew). Hopefully this will help the yeast culture acclimate a bit to the benzoic acid.
- I dumped the other 2 2L bottles of Mountain Dew into a stainless steel 8-qt pot and heated to boiling, whisking with a steel whisk frequently to help get the dissolved gases to bubble out (hopefully along with some benzoic acid in the steam). At this moment, it’s up to 75°C (about 165°F) according to the thermometer I have stuck in it. It’s steaming a bit, and I can smell some of the citrusy aroma boiling off, unfortunately. I was afraid that’d happen. UPDATE: our ancient stove with one working burner seems to have trouble getting this much liquid above 200°F without cranking it up all the way and risking the bottom getting too hot. Since there’s a substantial amount of hot water vapor coming off, I’m going to hope that’s carrying away some benzoic acid and just let it start cooling down. I’ll stir it vigorously and frequently with the whisk until the temperature drops to about 160°F and then I’ll cover it for the night.
- Meanwhile, before I go to bed, I’m going to recombine as much of the two yeast culture bottles as I can into one bottle, then rinse out the other. In that one, I’ll mix up about 12 ounces of tap water and enough corn sugar to reach a gravity of about 1.050-1.055. I’ll crush up another chewable vitamin and about half of the remaining arginine capsule into it, mix well, and warm it with a quick spin in the microwave (no exact measurements, just until it’s “obviously warm” to the touch after mixing). Then I’ll shake the V8 culture well to mix, and splash about a tablespoon’s worth into the new bottle.
 In the morning, I’ll re-aerate the new culture and add a tablespoon of corn sugar to wake it back up, then once it’s going, I’ll start adding the now-cooled cooked flat Mountain Dew to it in small increments. Assuming it keeps going, I’ll dump in the remainder of the L-Arginine capsule, crush in one last chewable vitamin, and them combine the new culture with the rest of the cooked flat Mountain Dew in a nice plastic 2-gallon “water” container that I picked up (already rinsed with iodophor and dried). Cap it with a latex glove attached with a rubber band as described in the “Pruno” entry in Leon Kania’s “Alaskan Bootlegger’s Bible” (Click image for link) just because I thought it was the funniest airlock design I’d ever run into. Yes, I am easily amused.
In the morning, I’ll re-aerate the new culture and add a tablespoon of corn sugar to wake it back up, then once it’s going, I’ll start adding the now-cooled cooked flat Mountain Dew to it in small increments. Assuming it keeps going, I’ll dump in the remainder of the L-Arginine capsule, crush in one last chewable vitamin, and them combine the new culture with the rest of the cooked flat Mountain Dew in a nice plastic 2-gallon “water” container that I picked up (already rinsed with iodophor and dried). Cap it with a latex glove attached with a rubber band as described in the “Pruno” entry in Leon Kania’s “Alaskan Bootlegger’s Bible” (Click image for link) just because I thought it was the funniest airlock design I’d ever run into. Yes, I am easily amused.- If I’m lucky, there’ll be so much live and active yeast in the second culture at that point that the fermentation will finish quickly, because I’m driving to Texas the following day. Alternatively, I may be lucky and the remaining benzoic acid will slow the yeast down, so I can safely leave it fermenting (sitting in my sink in case of overflow while I’m gone) for the week that I’ll be gone.
If I’m UNlucky, either it won’t ferment at all (and I can then use it to try to develop a Mountain Dew Tolerant strain of yeast), or it’ll ferment but taste utterly disgusting (in which case I can use it to try to obtain some Gluconobacter strains and make Mountain Dew Vinegar), or it’ll be “infected” and will already BE Mountain Dew Vinegar, which would also be pretty funny anyway. So, other than completely unforseen results, this experiment can’t be a TOTAL waste.
[Update 20080727: Preliminary results of this perverse project may be read In this more recent post…]
“They laughed at me! But I’ll show them all! AH, HAHAHAHA!”
 Another T-shirt to add to my list of T-shirts I want.
Another T-shirt to add to my list of T-shirts I want.
I’m spending more hours shoveling my way through the books and papers and crap we’ve got up here at House v1.0, since if all goes well I’ll be making a brief run back down to Southeast Texas so we can sign the papers for House v2.0 down there, at which point we’ll be able to start actually moving. I sure hope this one goes through. Not only is it our third attempt to buy a house down there, but I’ve already identified a convenient location to build my “Intentional Food Microbiology” brewlab in it.
Since there’s no way I can afford to buy a -80°F freezer, I have an obvious interest in alternate means of preserving the yeast, mold, and bacterial cultures that I want to keep. To me, drying seems like the most desirable method when it’s feasible, since dried cultures should require the least amount of maintenance. After a several-month delay, I’ve finally gotten around to getting back in touch with the archivist at Brewer’s Digest to see about getting an old article on the viability of dried yeast cultures[1].
Speaking of old but useful scientific papers, there’s an extremely nifty challenge going on through the month of May (deadline: May 31st) over at “Skulls in the Stars” blog: find a classic scientific paper, read it, and blog about it.
“My “challenge”, for those sciencebloggers who choose to accept it, is this: read and research an old, classic scientific paper and write a blog post about it. I recommend choosing something pre- World War II, as that was the era of hand-crafted, “in your basement”-style science. There’s a lot to learn not only about the ingenuity of researchers in an era when materials were not readily available, but also about the problems and concerns of scientists of that era, often things we take for granted now!”
I think this is a brilliant idea – the classic papers often seem to be forgotten and often explain things that people seem to take for granted these days. I already mentioned my post about the Gram Stain (original paper published in 1884), though that post really talks more about what has happened with the Gram Stain over the last 125 years rather than only being about the original paper. There are a couple of other classic microbiology papers that I’m going to try to get to if I have time before the May 31st deadline arrives.
I also need to get some yeast activated and get my must processed – I’m hoping a brief boil will reduce the amount of a yeast-inhibiting substance in it. I’ll post more detail after I get it going.
[1] Wickerham LJ, AND Flickinger MH:”Viability of yeast preserved two years by
the lyophile process.” 1946; Brewers Digest, 21, 55-59; 65.