Dr. Joseph over on the “(It’s a…) Micro World (…after all)” blog posted a list of his ten favorite microbes. After showing up in the comments of his post and being a wiseass about E.coli and Gram staining, the least I can do is participate here. Besides, it’s a great idea. Therefore here are ten of my current favorite microbes:
Category: Freakish Eukaryotes
I normally discuss NORMAL (prokaryotic) organisms on this blog, not perverted Eukaryotes.
Why “Perverted”? Well, as a society, we tend to think inter-SPECIES mating is pretty perverse, but eukaryotes derive from inter-PHYLUM mating…and an orgiastic one at that, according to my own pet hypothesis regarding how eukaryotes came to be…
Stir-Fried Random Ep 03: All I Want for Christmas Is…
 Thanksgiving = Shopping, evidently. Anyway, since this is about the time of year when the vast population of my devoted fans around the world begin demanding to shower me with gifts and asking what kinds of gifts they should give me…this episode of Stir-Fried Random has some suggestions. Enough suggestions, in fact, that I didn’t even have room to include a Nerd Word, Emprical Observation of the Week, or Microbiology Microlecture. Therefore, while this episode will probably be slightly less interesting to the microbiology and computer-nerd focussed listeners, it should be of special interest to members of my immediate family, secret admirers, cultists who worship me as a living embodiment of divine spiffitude, and agents of the NSA, FBI, CIA, USDA, and Federal Department of Blog Enforcement who are busily profiling me. There is some other stuff though – please give it a listen, pass copies along to your friends, play it over the Public Announcement system at school, turn it into a techno-dance remix video on YouTube®, whatever.
Thanksgiving = Shopping, evidently. Anyway, since this is about the time of year when the vast population of my devoted fans around the world begin demanding to shower me with gifts and asking what kinds of gifts they should give me…this episode of Stir-Fried Random has some suggestions. Enough suggestions, in fact, that I didn’t even have room to include a Nerd Word, Emprical Observation of the Week, or Microbiology Microlecture. Therefore, while this episode will probably be slightly less interesting to the microbiology and computer-nerd focussed listeners, it should be of special interest to members of my immediate family, secret admirers, cultists who worship me as a living embodiment of divine spiffitude, and agents of the NSA, FBI, CIA, USDA, and Federal Department of Blog Enforcement who are busily profiling me. There is some other stuff though – please give it a listen, pass copies along to your friends, play it over the Public Announcement system at school, turn it into a techno-dance remix video on YouTube®, whatever.
As usual, direct download links for mp3 and ogg versions, plus <audio> tag support for those with really new browsers to listen in place, and embedded Flash-based mp3 player for everyone else who wants to listen in the browser instead of downloading and singing along during your commute or whatever.
(UPDATE 20081126: I’ve REMOVED the embedded player for now – it seems it ignores me when I tell it to wait until it’s told to before it starts playing. Autoplay annoys the heck out of me, and this seems to insist on it. The embedded player will remain gone until I get it to behave properly. Meanwhile, you can double-click or “right-click -> save as:” on the ogg or mp3 download link to get the audio files to listen to. Apologies to anyone ambushed by the unwanted auto-playing of the sound…)
Show Notes:
Continue reading Stir-Fried Random Ep 03: All I Want for Christmas Is…
Yeast needs to breathe
 As readers may have guessed from previous posts, my brewing interests are minimally conventional. Fortunately, the Basic Brewing Radio podcast seems to regularly expand well beyond the usual “fermented malt flavored with a tisane of hops” thing (I need to try to make my own “Ginger Beer Plant” from scratch one of these days…). A couple of weeks ago, they did an episode covering an experiment on aeration methods which was very interesting. It does my ego good to know that I correctly guessed how the results would turn out. You can get a copy of the nice write-up of the experiment itself here, but here’s the simple version:
As readers may have guessed from previous posts, my brewing interests are minimally conventional. Fortunately, the Basic Brewing Radio podcast seems to regularly expand well beyond the usual “fermented malt flavored with a tisane of hops” thing (I need to try to make my own “Ginger Beer Plant” from scratch one of these days…). A couple of weeks ago, they did an episode covering an experiment on aeration methods which was very interesting. It does my ego good to know that I correctly guessed how the results would turn out. You can get a copy of the nice write-up of the experiment itself here, but here’s the simple version:
Aqua-pedestrianism and Ice Cream Yeast
 Today’s batch of blog-based Stir-Fried Random includes another interactive map of a lake-spanking expedition, a very brief musing on search engines, and a return to “intentional food microbiology” discussion. To preview: you can get pizza without ever getting out of the water on Lake Conroe, “spanking” is amusingly popular for search engines, and no, there is not normally any yeast in ice cream, but perhaps there could be. Read on, please…
Today’s batch of blog-based Stir-Fried Random includes another interactive map of a lake-spanking expedition, a very brief musing on search engines, and a return to “intentional food microbiology” discussion. To preview: you can get pizza without ever getting out of the water on Lake Conroe, “spanking” is amusingly popular for search engines, and no, there is not normally any yeast in ice cream, but perhaps there could be. Read on, please…
Benzoic Acid Part 2: “Sour Stuff”
Okay, now that the boring review is over with…
Consider the cell. It doesn’t matter what kind of cell – bacterial, archael, fungal, animal, whatever. It’s still a tiny droplet of slightly salty water, thickened by a bunch of enzymes, other proteins, and various other substances floating around in the water. There’s also one other component that makes this a “cell” rather than soup: a bubble made of fatty material that the droplet is wrapped in, called the cell membrane. Depending on what kind of cell you’re thinking of, there may or may not be a “cell wall” made of some sort of rigid material, with the cell membrane inside of it. There may also be more than one membrane as is the case with the classic “Gram negative” style of bacterium, which has a second “outer” membrane wrapped around its cell wall. If it’s a eukaryotic cell, it’ll even have tiny little “organelles” inside itself wrapped in their own little membranes…but whatever. It’s the innermost one, inside of whatever cell wall may be there but wrapped around the cell’s guts, that we’re concerned with here.
Since stuff that will dissolve readily in water doesn’t tend to dissolve well into fats, and vice-versa, the cell membrane not only prevents stuff dissolved in the water inside the cell from leaking out, it also prevents stuff in the water outside from getting in. This lets a cell maintain itself at near neutral pH even if it happens to live in a very acidic environment, or an appropriate level of, say, sodium salts even if it lives in the Great Salt Lake.
This brings us back again to benzoic acid, which you should recall from the previous post alternates between a dissociated hydrogen-ion-and-benzoate-ion form and a combined, netural form in water. You may have noticed that foods preserved with benzoates tend to be sour, like fruit juices or soda. That’s because “sour” is the flavor of acid, and benzoic acid’s ability to be a preservative is only good in acidic environments
Useless Knowledge Break: the German word for acid is “Saurstoff”. Yes, that is pronounced like “sour stuff”, and no, that is not a coincidence.
An acidic environment means lots of extra hydrogen ions (“protons”) floating around. That also means that when a molecule of benzoic acid splits into a hydrogen ion and benzoate ion, it takes less time before another hydrogen ion comes by and the molecule can recombine again and therefore a bigger majority of the benzoate floating around at any moment is in the combined, somewhat fat-soluble neutral form. In that form, it can soak into a cell membrane if it encounters one.
If that molecule drifts through the membrane and gets to the inside of the cell, it may touch the less acidic watery environment there and dissociate into ions again and be unable to return through the membrane. The released hydrogen ions mean the inside of the cell becomes more acidic. As of today (20080806), the Wikipedia entry for Sodium Benzoate cites a single paper from the early 1980’s saying that when the inside of a yeast cell gets acidic enough, it prevents a specific step in the energy-generating process from working. This may be true, but there’s more to the story than this.
Obviously the membrane can’t totally seal the cell off from the outside, or the cell would be unable to excrete wastes or take in food molecules, so there are numerous specialized “transport” proteins that stick through the membrane to allow specific kinds of molecules in and out. Lots of biochemical reactions release hydrogen ions, so there are transport proteins that can shove hydrogen ions out of the cell and into the cell’s surroundings. The problem is that all substances naturally diffuse from areas of higher concentration to areas of lower concentration, so in an acidic environment the natural direction that hydrogen ions “want” to flow is into the more neutral cell. These transport proteins can shove the hydrogen ions in the opposite direction, but like pushing a boulder uphill it costs energy. This seems to be the primary reason that benzoic acid prevents bacteria and yeasts from growing – it makes them waste energy that they would be using for growth just to keep taking the hydrogen ions that the benzoic acid helps leak in through the cell membrane and shoving them back outside. The figure above is linked to a page at Helsinki university that discusses this type of preservative action in more detail.
Simple and elegant, and this seems to have been assumed to be the whole explanation for some time. But what happens to the benzoate ion when its hydrogen ion gets pumped away? Does it do anything?
Coming up next: Endocannibalism!
Eggs suck.
Don’t misunderstand – I like all kinds of foods made with eggs. Eggs are tasty. They’re handy. They’re nutritious, too. Their protein is so good they are the standard against which nutritionists have historically compared other food proteins. As a bonus, a medium-sized egg contains only one-eighth the cholesterol in a single ounce of human brain (and is much less likely to give you kuru).
However, could it be possible to come up with a more inconvenient packaging scheme? This thing was obviously designed by someone that hates us. “Let’s see, we’ll make it tasty and very nutritious. But just so people don’t think we like them, we’ll make it the consistency of snot, and package it by sealing it inside a specially-made, inedible brittle container so that you have to literally smash the thing open to get at the food, ensuring that the consumer* gets nasty goo all over his or her hands, or gets shards of the container mixed in with the food, or both. That’ll show the little jerks. And if that’s not enough, we’ll also have that container extruded out of a chicken’s butt.” (For some odd reason, as I write this, I’m picturing an Evil Santa Claus giving a presentation to the Evil Elves who are about to go off and implement this idea…”Sneezy, Drippy, and Runny – you three will head up the design committee…”)
They’re not screw flies or anything, but perhaps eggs can still count as an example of “irreducible grotesqueness”. (The picture, in case it isn’t obvious, is an egg separator. The image is linked to the site where you can buy them.)
This short rant has been brought to you by I’m-Making-Too-Many-Egg-Based-Meals-Lately Industries. That, and a second test to make sure I’ve got the DNS issues that I initially had resolved now. Can you all see this? I’d be thankful if everyone who was seeing this would post a quick “yeah, I see it” in the comments so that I can get on with the real posts again.
* Am I the only one who finds it insulting to be called a “consumer”, as though I were nothing more than a gaping mouth with a wallet? Am I sitting here mouth agape like a baby bird, waiting for a “supplier” to stroll by, grab some money out of my wallet, and cough up some “product” for me to “consume”? (I contend that I am not a “consumer” but a active participant in this economy, dagnabbit!)
(Tap Tap Tap) Is this thing on?…
Move is underway. Here’s a quick update / test.

On the way home (to Texas, that is, with a load of furniture from House v1.0 in Idaho) we adopted a co-dog. Now Cornelia isn’t the only official dog in the house.
Yeah, I know, pretty frivolous stuff. What do you want – this is mainly a test post. You guys ARE seeing this post, right? (Please comment and let me know). I’ve finally found and jumped through the hoops necessary to migrate the bigroom.org website over to the new host. It’s also running on the old server in House v1.0 on the DSL line as well, but this post isn’t getting put on it. If I’ve done everything correctly, hosting for bigroom.org should now be handled by Eskimo North. Just a few other minor tweaks and I’ll be set to return to House v1.0 and shut down the old server so I can move it down here to Texas.
I’ve gone from about 4400ft elevation down to about 250ft, but the plastic bottles of Mountain Dew® Wine seem to have positive pressure, so it would appear there is still some live yeast left in there and the priming sugar is slowly doing its job. I’m pretty sure the benzoic acid is what’s slowing down the activity so drastically, but it hasn’t killed it off yet.
I’ve also got a couple of entries in mind already for the next The Giants’ Shoulders blog carnival, and hopefully some more crazed brewing project news to offer sometime. And, of course, the promised post on why benzoic acid works. Stay tuned.
Mountain Dew® Wine: Disappointment strikes!
<whine excuse=”obligatory”>Have I ever mentioned what a huge hassle it is to relocate from one abode to another 1600 miles away?…</whine>
 I’m finally back at House v1.0 where I can check on the progress of my Mountain Dew® Wine. It appears to have managed to ferment, in spite of the severe dose of preservatives in the stuff designed to prevent that from happening. It went somewhat slowly, but it’s gone from an original gravity of around 1.054 down to about 1.011 or so, suggesting about, say, 5-6% alcohol in the final product, which has faded to a pale, cloudy yellow color. Hopefully the cloudiness is from still-living yeast, which has now demonstrated that it is reasonably benzoic-acid-and-caffeine tolerant.
I’m finally back at House v1.0 where I can check on the progress of my Mountain Dew® Wine. It appears to have managed to ferment, in spite of the severe dose of preservatives in the stuff designed to prevent that from happening. It went somewhat slowly, but it’s gone from an original gravity of around 1.054 down to about 1.011 or so, suggesting about, say, 5-6% alcohol in the final product, which has faded to a pale, cloudy yellow color. Hopefully the cloudiness is from still-living yeast, which has now demonstrated that it is reasonably benzoic-acid-and-caffeine tolerant.
I fear I must report that the result is a crushing disappointment to me. It’s not very good. Worse yet, it’s not very bad, either. I was hoping that if it wasn’t surprisingly tasty that it would at least be shockingly awful in some interesting way so I’d have something entertaining to say about it here.
Actually, the adjective that comes to mind is “inoffensive”. If you would like to whip up a quick simulation of what I’ve got here, you might be able to do it like this: Take some citrus-flavored sparkling mineral water. Dilute it about half with (uncarbonated) distilled water. Then mix about 7 volumes of that with one volume of vodka. What I’ve got here is slightly sparkling, with a barely noticeable citrus flavor and little or no remaining sweetness. It’s a little surprising to me just how much of the flavor of Mountain Dew® apparently comes from its sweetness. Perhaps next time I try this (if there ever IS a “next time”…) I’ll have to mix regular and “diet” Mountain Dew® – with some bonus sugar to make up the difference, of course.
I’m not completely done here. I’m still going to dispense it into cleaned bottles with a little bit of sugar to prime it for full carbonation. Sanitized plastic soda-bottles of course – none of that snobby glass stuff for this here experimental drinkin’ substance! It may be high-class for “pruno”, but it’s sure as heck not Champagne™. (Besides, I want to evaluate reusing plastic bottles anyway – it’d be a lot easier to tell when there’s too much pressure and to let some of the pressure off if there is.) Plus, I need to take some of the still-live yeast and keep it alive. No point in developing a benzoic-acid-tolerant yeast strain and not keeping it!
On a related note, an article was mentioned on fark.com, saying that back in 1955, a scientist “proved” that it is not normally possible to get drunk on beer. Of course, he seems to have been referring to dilute mass-market bladderwash and his reasoning was that a typical human stomach cannot contain enough 3.7%-alcohol beer for a typical human to achieve a dangerous blood-alcohol level.
As usual, the articles (see here and here) go for the “a scientist says this” part but never bother to say WHERE the scientist says it – usually a real scientific publication.
A quick search of pubmed turns up a likely candidate:
Greenberg LA: “The definition of an intoxicating beverage.” Q J Stud Alcohol. 1955 Jun;16(2):316-25 (link goes to the pubmed entry, which has little more information that this).
I do believe it is a moral imperative that I get a copy of this article somewhere so that I may reference it later. Is there anyone out there reading this who might be able to get a copy of this paper somewhere for me? Please?…
SHENANIGANS! Caffeine is our FRIEND!
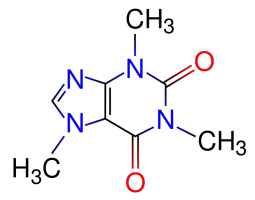 Our new Asylum has real internet finally now and we’re getting settled in. The Houston area here is one of the most hot and humid areas of the US. All hot and sweaty. So of course I’ve been advised that my favorite psychotropic substance – 1,3,7-trimethylxanthine [“caffeine” for party-poopers who aren’t into the fancier names] – is no longer my friend, because it’s a diuretic that’ll dehydrate me, right?
Our new Asylum has real internet finally now and we’re getting settled in. The Houston area here is one of the most hot and humid areas of the US. All hot and sweaty. So of course I’ve been advised that my favorite psychotropic substance – 1,3,7-trimethylxanthine [“caffeine” for party-poopers who aren’t into the fancier names] – is no longer my friend, because it’s a diuretic that’ll dehydrate me, right?
NO! Shenanigans! Caffeine is our FRIEND! And that stuff about it being a diuretic? CRAP! LIES AND SLANDER!
But don’t just take my word for it. After all, humans are a bunch of freakish multicellular soft-celled eukaryotes, and I normally focus on normal organisms like bacteria, archaea, and yeasts. So, let’s ask some real human-physiology type scientists and check out their official peer-reviewed findings:
Armstrong LE, Pumerantz AC, Roti MW, Judelson DA, Watson G, Dias JC, Sokmen B, Casa DJ, Maresh CM, Lieberman H, Kellogg M: “Fluid, electrolyte, and renal indices of hydration during 11 days of controlled caffeine consumption.”; Int J Sport Nutr Exerc Metab. 2005 Jun;15(3):252-65.
“[…]The following variables were unaffected (P > 0.05) by different caffeine doses on days 1, 3, 6, 9, and 11 and were within normal clinical ranges: body mass, urine osmolality, urine specific gravity, urine color, 24-h urine volume, 24-h Na+ and K+ excretion, 24-h creatinine, blood urea nitrogen, serum Na+ and K+, serum osmolality, hematocrit, and total plasma protein. Therefore, C0, C3, and C6 exhibited no evidence of hypohydration.[…]”
Armstrong LE, Casa DJ, Maresh CM, Ganio MS: “Caffeine, fluid-electrolyte balance, temperature regulation, and exercise-heat tolerance.” Exerc Sport Sci Rev. 2007 Jul;35(3):135-40.
“[…]This review, contrary to popular beliefs, proposes that caffeine consumption does not result in the following: (a) water-electrolyte imbalances or hyperthermia and (b) reduced exercise-heat tolerance.”
(Review article, apparently – Abstract on Pubmed)
Del Coso J, Estevez E, Mora-Rodriguez R: “Caffeine effects on short-term performance during prolonged exercise in the heat.” Med Sci Sports Exerc. 2008 Apr;40(4):744-51.
“[…]RESULTS: Without fluid replacement (NF and NF + CAFF), subjects were dehydrated by 3.8 +/- 0.3%[…]CONCLUSION: During prolonged exercise in the heat, caffeine ingestion (6 mg.kg body weight) maintains MVC and increases PMAX despite dehydration and hyperthermia. When combined with water and carbohydrate, caffeine ingestion increases maximal leg force by increasing VA (i.e., reducing central fatigue).”
(“NF” = “No Fluid replacement” – the “dehydration” mentioned here is due to exercising in the heat, and doesn’t appear to be related to whether the test subjects consumed caffeine or not)
Scott D, Rycroft JA, Aspen J, Chapman C, Brown B:”The effect of drinking tea at high altitude on hydration status and mood.” Eur J Appl Physiol. 2004 Apr;91(4):493-8. Epub 2004 Feb 11.
“[…]Several markers of hydration status were also taken immediately pre and post each condition, including measures of urine specific gravity, urine electrolyte balance (K+, Na+), and urine colour. None of these measures indicated a difference in hydration status as a result of the dietary intervention in either the control or tea condition.[…]”
(In this study, the tea was the only caffeine-containing substance involved. The study group’s caffeine came solely from the tea. The control group got no caffeine at all.)
Paluska SA: “Caffeine and exercise.” Curr Sports Med Rep. 2003 Aug;2(4):213-9.
“[…]It[caffeine] is relatively safe and has no known negative performance effects, nor does it cause significant dehydration or electrolyte imbalance during exercise.[…]”
Grandjean AC, Reimers KJ, Bannick KE, Haven MC.: “The effect of caffeinated, non-caffeinated, caloric and non-caloric beverages on hydration.” J Am Coll Nutr. 2000 Oct;19(5):591-600.
“[…]This preliminary study found no significant differences in the effect of various combinations of beverages on hydration status of healthy adult males.[…]”
Pubmed entry – full text available
See? Oh, I know what you’re going to say next – “But, like, dude! When I drink my Venti Mocha Crappucino [note: Link goes to “Foamy the Squirrel”, who is a bit of a pottymouth, ranting about the “Tall/Grande/Venti” nonsense. It amused me.] or a can of Jolt Ultra I have to take a major whiz a little while later! Isn’t that ‘cuz of the caffeine?” Well, no, it isn’t. It’s because you just drank a bunch of liquid. Duh.
So, you see, caffeine really is our friend. Be nice to caffeine. But don’t feed it to your yeast in the presence of benzoic acid because it’ll kill them. See? I managed to turn this into a segue back to the stuff I was talking about before the whole “buy a house in Texas” thing started interfering. Stay tuned…
Very brief post…
Sorry about having another brief bout of blogstipation. We’ve finally managed to close on a house and now we’re probationary Texans (y’all). I’ve been spending the last week+ driving back to SE Idaho (by way of Best Friends, since we now have room to hire a second dog to hopefully keep Cornelia the Laser Dog company. I’ve got to lug 3 cats (and one goldfish in a small fish tank) a distance of 1600 miles or so starting in about 6-8 hours. Wish me (good) luck.
After the weekend, I should have some time to get back to the real posts. The Mountain Dew Wine was virtually unfermented when I returned, even after sitting there 10 days, but since I’ve gotten back it’s started going. Still slowly – the bubbler spits out 3-5 bubbles ever 40 seconds or so – but it’s going. It’ll be interesting to see what I end up with. I wanted to do 2-3 posts on the effects of benzoic acid on yeasts (that’s the preservative in Mountain Dew®), and I would swear I had one or two others in mind. Oh, yes, and an update on getting a Magellan GPS replacement that can actually be used – they seem to have located a slightly lower-end model eXplorist that they can send me. I sent them back their “walled garden”-based Triton 500, so the replacement unit ought to show up next week, I think. Their service has been pretty good, at least.
